Hydrocyanic acid,HCN,is a weak acid.Write a net ionic equation for the reaction of aqueous hydrocyanic acid and aqueous sodium hydroxide.
A) HCN(aq) + NaOH(aq) Na+(aq) + CN-(aq) + H2O( 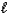 )
)
B) HCN(aq) + H2O(aq) CN-(aq) + H3O+(aq)
C) H+(aq) + OH-(aq) H2O( 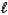 )
)
D) HCN(aq) + OH-(aq) CN-(aq) + H2O( 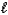 )
)
E) H+(aq) + NaOH(aq) Na+(aq) + H2O( 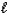 )
)
Correct Answer:
Verified
Q12: A precipitate will form when aqueous nickel(II)chloride
Q17: A precipitate will form when aqueous Pb(NO3)2
Q33: If an aqueous solution of _ is
Q38: Write a balanced chemical equation for
Q41: Which of the following is a
Q43: What is the balanced equation for carbonate
Q44: Which of the following is a weak
Q45: What are the spectator ions in the
Q47: Which of the following is a
Q50: Which of the following compounds will produce
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents