All of the following are oxidation-reduction reactions EXCEPT
A) CaCO3(s) CaO(s) + CO2(g)
B) 2 Na(s) + Br2(g) 2 NaBr(g)
C) Fe(s) + 2 HCl(aq) FeCl2(aq) + H2(g)
D) 2 C(s) + O2(g) 2 CO(g)
E) 2 H2O( 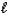 ) 2 H2(g) + O2(g)
) 2 H2(g) + O2(g)
Correct Answer:
Verified
Q64: Which of the following elements generally acts
Q65: Ionic and molecular compounds that form ions
Q67: If an aqueous sodium hydroxide solution is
Q71: _ acid is produced in a larger
Q72: What is the oxidation number of
Q74: Which molecule in the reaction below
Q75: Write a balanced net ionic equation
Q76: Which of the following chemical equations
Q78: What is the oxidation number of each
Q79: What is the oxidation number of each
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents