Aspirin is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride
(M = 102.1 g/mol) .C7H6O3(s) + C4H6O3( 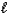 ) C9H8O4(s) + C2H4O2(
) C9H8O4(s) + C2H4O2( 
)
If 2.04 g of C9H8O4 (M = 180.2 g/mol) is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
A) 28.8%
B) 29.0%
C) 50.9%
D) 51.6%
E) 67.3%
Correct Answer:
Verified
Q16: Calculate the number of moles of O2
Q17: If the complete combustion of an
Q18: Potassium hydrogen carbonate decomposes with heat
Q19: Aspirin (C9H8O4)is produced by the reaction
Q20: One step in the isolation of pure
Q22: Complete combustion of a 0.30-mol sample of
Q24: Of the following,the only empirical formula is
A)
Q25: Sulfur hexafluoride is produced by reacting
Q26: Chlorine was passed over 2.02 g of
Q36: A 1.850 g mixture of SrCO3 and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents