Which of the following sets of quantum numbers is allowed?
A) n = 2, 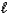 = 1,
= 1, 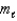 = +1/2,ms = -1/2
= +1/2,ms = -1/2
B) n = 3, 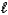 = 2,
= 2, 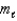 = +1,ms = +1
= +1,ms = +1
C) n = 4, 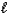 = 1,
= 1, 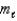 = 0,ms = -1/2
= 0,ms = -1/2
D) n = 4, 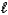 = 3,
= 3, 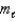 = -1,ms = 0
= -1,ms = 0
E) n = 5, 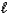 = 2,
= 2, 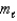 = +2,ms = -1
= +2,ms = -1
Correct Answer:
Verified
Q15: Which of the following statements concerning ground
Q16: How many electrons can be described by
Q17: Which element is found in the p-block
Q18: How many electrons can be described by
Q19: Which of the following statements is true
Q21: What is the ground-state electron configuration of
Q22: Which of the following electron configurations corresponds
Q23: Hund's rule states that the most stable
Q24: The complete electron configuration of tin is
A)
Q26: Which atom has the ground state electronic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents