Hund's rule states that the most stable arrangement of electrons (for a ground state electron configuration)
A) has a filled valence shell of electrons.
B) has three electrons per orbital,each with identical spins.
C) has 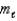 values greater than or equal to +1.
values greater than or equal to +1.
D) has the maximum number of unpaired electrons,all with the same spin.
E) has two electrons per orbital,each with opposing spins.
Correct Answer:
Verified
Q18: How many electrons can be described by
Q19: Which of the following statements is true
Q20: Which of the following sets of quantum
Q21: What is the ground-state electron configuration of
Q22: Which of the following electron configurations corresponds
Q24: The complete electron configuration of tin is
A)
Q26: Which atom has the ground state electronic
Q26: Which of the following orbital diagrams
Q27: Which element has the following ground state
Q28: Which element has the following ground state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents