The ground-state electron configuration of a 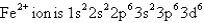 .Therefore,
.Therefore, 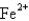 Is
Is
A) paramagnetic with four unpaired electrons.
B) diamagnetic.
C) paramagnetic with one unpaired electron.
D) paramagnetic with three unpaired electrons.
E) paramagnetic with two unpaired electrons.
Correct Answer:
Verified
Q40: What is the ground state electron configuration
Q50: Which of the following equations corresponds to
Q51: What 2+ ion has the following ground
Q52: An atom of which of the following
Q54: What is the ground-state electron configuration of
Q57: What 2- ion has the following ground
Q58: The change in energy for which of
Q59: In general,atomic radii
A) decrease down a group
Q60: Rank the following atoms in order decreasing
Q66: An atom of which of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents