Which of the following species would you expect to have the largest radius?
A) 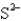
B) P
C) 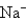
D) 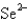
E) 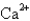
Correct Answer:
Verified
Q57: What 2- ion has the following ground
Q58: The change in energy for which of
Q59: In general,atomic radii
A) decrease down a group
Q60: Rank the following atoms in order decreasing
Q62: The change in energy for the
Q62: Place the following ions in order from
Q63: What is the charge formed by alkaline
Q66: An atom of which of the following
Q66: A metal halide forms when strontium reacts
Q89: According to the general trend in electron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents