Using the following data reactions: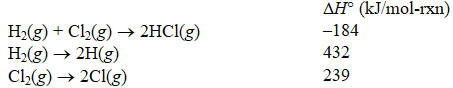
Calculate the energy of an H-Cl bond.
A) 92 kJ/mol
B) 855 kJ/mol
C) 487 kJ/mol
D) 428 kJ/mol
E) 244 kJ/mol
Correct Answer:
Verified
Q81: Use Lewis structures to predict the bond
Q83: Use the bond energies provided to complete
Q83: The molecular geometry of a molecule whose
Q84: Based on the following data,what is the
Q84: In benzene,C6H6,the six carbon atoms are arranged
Q85: Which of the following species has the
Q88: Use Lewis structures to predict the bond
Q88: If a molecule has a positive and
Q89: Using bond-energy data,what is is
Q91: What is the bond order of O22+?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents