A gas occupying a volume of 1.50 L exerts a pressure of 700 mmHg at 200°C.Which mathematical expression gives the correct pressure at 6.00 L and 400°C?
A) 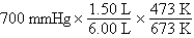
B) 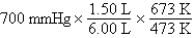
C) 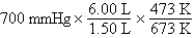
D) 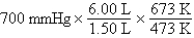
E) 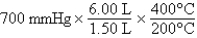
Correct Answer:
Verified
Q31: You have 49.1 g of O2 gas
Q32: The volume of a sample of gas
Q33: What volume of oxygen will react
Q34: A 45.0 L gas cylinder contains
Q35: What volume is occupied by 35.0
Q37: The pressure of 5.4 L of nitrogen
Q38: Gaseous chlorine is held in two separate
Q39: Which of the following concerning the behavior
Q40: What is the pressure of a
Q41: A mixture of KCl and KClO3 weighing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents