One way to isolate metals from their ores is to react the metal oxide with carbon as shown in the following reaction (M = metal) : 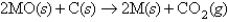
If 34.14 g of a metal oxide reacted with excess carbon and 4.38 L of CO2 formed at 100°C and 1.50 atm,what is the identity of the metal?
A) Hg
B) Mg
C) Cu
D) Zn
E) Cd
Correct Answer:
Verified
Q41: A mixture of KCl and KClO3 weighing
Q42: Into a 2.22-liter container at 25°C are
Q43: The following equation represents the partial
Q44: The density of ethane,C2H6 (30.1 g/mol),at 32°C
Q45: An excess of sodium hydroxide is treated
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents