Water can be decomposed by electrolysis into hydrogen gas and oxygen gas.What mass of water must decompose to fill a 3.00 L flask to a total pressure of 2.00 atm at 298 K with a mixture hydrogen and oxygen? (R = 0.08206 L.atm/mol.K)
2 H2O( 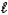 ) 2 H2(g) + O2(g)
) 2 H2(g) + O2(g)
A) 1.47 g
B) 2.95 g
C) 4.42 g
D) 6.63 g
E) 8.84 g
Correct Answer:
Verified
Q57: Ammonia gas is synthesized according to
Q58: A 3.74 gram sample of a certain
Q59: What volume does 40.5 g of N2
Q60: Aqueous hydrochloric acid reacts with magnesium
Q61: What is the volume occupied by
Q63: What is the total volume of
Q65: An unknown gaseous hydrocarbon contains 85.63% C.Its
Q66: A 22.4 L high pressure reaction
Q67: The rate of effusion of an unknown
Q90: At STP,as the molar mass of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents