The rate of effusion of an unknown gas was measured and found to be 14.5 mL/min.Under identical conditions,the rate of effusion of pure oxygen (O2) gas is 17.0 mL/min.Based on this information,the identity of the unknown gas could be:
A) 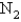
B) 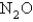
C) 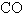
D) 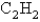
E) none of these
Correct Answer:
Verified
Q62: Water can be decomposed by electrolysis
Q63: What is the total volume of
Q64: How long will it take 10.0 mL
Q65: An unknown gaseous hydrocarbon contains 85.63% C.Its
Q66: A 22.4 L high pressure reaction
Q68: Which of the following statements are postulates
Q69: A vessel with a volume of 26.9
Q70: The partial pressures of CH4,N2,and O2 in
Q78: The layer of the atmosphere closest to
Q90: At STP,as the molar mass of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents