The Heat of Vaporization of Benzene,C6H6,is 30 CHow Much Energy in the Form of Heat Is Required
The heat of vaporization of benzene,C6H6,is 30.7 kJ/mol at its boiling point of 80.1 C.How much energy in the form of heat is required to vaporize 148 g benzene at its boiling point?
A) 0.207 kJ
B) 4.82 kJ
C) 16.2 kJ
D) 58.2 kJ
E) 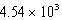 kJ
kJ
Correct Answer:
Verified
Q19: As pure molecular solids,which of the following
Q23: On a relative basis,the weaker the intermolecular
Q38: Which intermolecular force or bond is responsible
Q39: What intermolecular force or bond is primarily
Q41: Which of the following liquids has
Q44: Xenon has an enthalpy of vaporization
Q45: Mount Everest rises to a height
Q46: A liquid has an enthalpy of vaporization
Q48: A particular compound has an enthalpy of
Q54: In a certain mountain range,water boils at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents