Using the thermodynamic data below,and a value of -717 kJ/mole for the lattice enthalpy for KCl,calculate the ionization energy of K.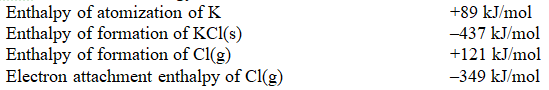
A) -576 kJ/mol
B) +141 kJ/mol
C) +419 kJ/mol
D) +576 kJ/mol
E) +597 kJ/mol
Correct Answer:
Verified
Q25: Which of the following compounds is expected
Q29: Which of the following is expected to
Q32: Iron(II)sulfide has a primitive cubic unit cell
Q52: Silver chloride crystallizes with the sodium chloride
Q53: Calculate the lattice energy of NaBr(s),given
Q54: Lattice enthalpy may be calculated using the
Q57: Elements that have their highest energy
Q58: Which two of the following materials are
Q60: The lattice energy of NaBr is
Q61: Which of the statements concerning the phase
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents