Assume the Reaction Below
2 NO(g)+ O2(g) 2 NO2(g)
Proceeds Via the Following Rate Expression:
Which
Assume the reaction below
2 NO(g) + O2(g) 2 NO2(g)
Proceeds via the following rate expression: 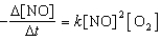
Which of the following statements concerning the above chemical reaction and rate equation is/are CORRECT?
1) The reaction is second-order with respect to NO.
2) The rate of disappearance of O2 is two times the rate of appearance of NO2.
3) According to the balanced chemical equation,the reaction is fifth-order overall.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3
Correct Answer:
Verified
Q2: What is the name given to a
Q5: Which statement concerning relative rates of
Q6: Which of the following conclusions concerning the
Q7: For the reaction provided,the rate of
Q8: Given the initial rate data for
Q10: The following data were obtained in
Q11: Given the initial rate data for
Q12: What is the overall order of
Q13: Which of the following expressions does
Q14: For a certain overall third-order reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents