The reactants A and B are mixed,and the reaction is timed until a color change occurs.The data are as follows: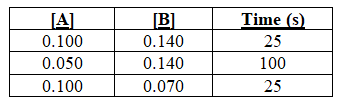
The order of the reaction with respect to reactant A is
A) 2.
B) 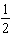 .
.
C) 1.
D) 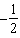 .
.
E) 0.
Correct Answer:
Verified
Q3: How are the exponents in a rate
Q14: For a certain overall third-order reaction
Q15: Consider the exothermic combustion of coal.Which of
Q16: The rate law for a reaction
Q17: Which relationship correctly compares the rates
Q18: The average rate of disappearance of ozone
Q20: If a reaction is third-order with respect
Q22: For the first-order decomposition of N2O5
Q23: Given the initial rate data for
Q24: In a first-order reaction,the half-life is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents