For the reaction TlSCN(s)  Tl+(aq) + SCN-(aq) ,Kc =
Tl+(aq) + SCN-(aq) ,Kc = 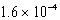
At 25 C.Which of the following concerning a 125 mL solution containing 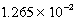
M Tl+, 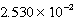
M SCN- and a large excess of TlSCN(s) is/are correct?
1) The mixture is at equilibrium.
2) Additional TlSCN(s) must precipitate to attain equilibrium.
3) The reaction quotient (Q) is greater than one.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3
Correct Answer:
Verified
Q12: If the reaction quotient,Q,is greater than K
Q22: At a high temperature,equal concentrations of 0.160
Q23: Consider the reaction
A(aq) 
Q24: Exactly 1.0 mol N2O4 is placed in
Q25: Which of the following is always
Q28: At a given temperature,0.0664 mol N2O4(g)is
Q29: Consider the following reaction: Q30: At 25 Q31: The reaction quotient,Q,for a system is Q39: Which of the following statements about the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents