Excess Ag2SO4(s) is placed in water at 25 C.At equilibrium,the solution contains 0.029 M Ag+(aq) .What is the equilibrium constant for the reaction below?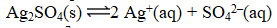
A) 1.8 10-7
B) 6.1 10-6
C) 1.2 10-5
D) 2.4 10-5
E) 8.4 10-4
Correct Answer:
Verified
Q5: If the reaction quotient,Q,is equal to K
Q28: At a given temperature,0.0664 mol N2O4(g)is
Q29: Consider the following reaction: Q30: At 25 Q31: The reaction quotient,Q,for a system is Q32: An aqueous mixture of phenol and ammonia Q34: A 2.5 L flask is filled with Q35: A 10.0-g sample of solid NH4Cl is Q37: When 0.20 mole HF is dissolved Q38: Consider the following equilibrium:![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents