Which equation depicts aqueous hydrogen sulfide behaving as a Brønsted-Lowry acid in water?
A) H2S(aq) + 2 OH-(aq) 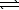 SO2(aq) + 2 H2(g)
SO2(aq) + 2 H2(g)
B) H2S(aq) + H3O+(aq) 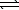 H3S+(aq) + H2O(
H3S+(aq) + H2O(  )
)
C) H2S(aq) + H2O(  )
) 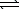 HS-(aq) + H3O+(aq)
HS-(aq) + H3O+(aq)
D) HS-(aq) + H3O+(aq) 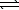 H2S(aq) + H2O(
H2S(aq) + H2O( 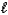 )
)
E) H2S(aq) + H2O( 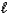 )
) 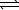 H3S++(aq) + OH-(aq)
H3S++(aq) + OH-(aq)
Correct Answer:
Verified
Q4: Which of the following substances is never
Q5: Molecules or ions that can alternately behave
Q6: What is the conjugate base of [Fe(H2O)6]3+(aq)?
A)
Q7: Which of the following species is amphiprotic
Q7: Which of the following substances is never
Q8: The autoionization of pure water,as represented
Q9: Which equation depicts hydrogen phosphate ion behaving
Q10: Which of the following pairs of species
Q11: What is the conjugate
Q13: In the following reaction,
HCO3-(aq)+ H2O( 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents