In the following reaction,
HCO3-(aq) + H2O( 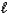 )
) 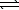 CO32-(aq) + H3O+(aq)
CO32-(aq) + H3O+(aq)
A) H3O+ is an acid and HCO3- is its conjugate base.
B) HCO3- is an acid and CO32- is its conjugate base.
C) HCO3- is an acid and H2O is its conjugate base.
D) H2O is an acid and CO32- is its conjugate base.
E) H3O+ is an acid and CO32- is its conjugate base.
Correct Answer:
Verified
Q6: What is the conjugate base of [Fe(H2O)6]3+(aq)?
A)
Q7: Which of the following substances is never
Q8: The autoionization of pure water,as represented
Q9: Which equation depicts hydrogen phosphate ion behaving
Q10: Which of the following pairs of species
Q10: Which equation depicts aqueous hydrogen sulfide behaving
Q11: What is the conjugate
Q16: Which of the following equations shows that
Q17: At 50°C the autoionization constant for
Q18: Which of the following expressions is not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents