Given that Ka for the weak acid HA is 3.46 10-8,calculate K for the reaction of HA with OH-.HA(aq) + OH-(aq) . A-(aq) + H2O( 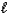 )
)
A) 3.46
B) 3.46 106
C) 3.46 10-22
D) 2.89 1021
E) 2.89 10-7
Correct Answer:
Verified
Q54: At 25
Q55: Given the following equilibrium constants,
Ka (HSO4-)=
Q56: At 25
Q57: What is the equilibrium hydronium ion concentration
Q58: Which acid-base reaction results in an
Q60: Given the equilibrium constants for the
Q61: What is the hydroxide-ion concentration of
Q62: Calculate the pH of a 0.04
Q62: All of the following compounds are acids
Q63: What is the pH of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents