The chemical equations below show the reaction of Al(OH)3 as a Lewis acid and as a Lewis base,respectively.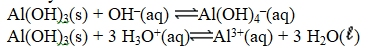
Substances that can behave as either Lewis acids or bases are called ________ substances.
Correct Answer:
Verified
Q26: Which of the following is the strongest
Q28: Rank H3PO4,H2PO4-,and HPO42- in order of increasing
Q78: What is the pH of the
Q79: What is the pH of a
Q80: What is the pH of a
Q84: In the reaction
CuO(s)+ CO2(g)
Q86: When a Lewis acid and a Lewis
Q87: A molecule that can behave as either
Q88: Which of the following species
Q89: Which of the following molecules or ions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents