An impure sample of sodium carbonate,Na2CO3,is titrated with 0.113 M HCl according to the reaction below.
2 HCl(aq) + Na2CO3(aq) 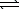 CO2(g) + H2O(
CO2(g) + H2O( 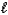 ) + 2 NaCl(aq)
) + 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
A) 0.295%
B) 15.7%
C) 25.5%
D) 51.1%
E) 67.9%
Correct Answer:
Verified
Q45: A 25.0 mL sample of 0.200
Q46: The concentration of magnesium carbonate in a
Q47: A 50.00-mL solution of 0.0729 M
Q48: The hydroxide ion concentration of a saturated
Q51: A solution containing 10.mmol of CO32- and
Q52: Which is the best colored indicator
Q53: What color change is exhibited by phenolphthalein
Q54: What is the solubility product expression for
Q55: Potassium hydrogen phthalate (KHP)is used to standardize
Q121: Which of the following indicators is most
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents