The solubility of barium carbonate (BaCO3) in water at 25°C is 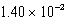 g/L.What is the Ksp of this sparingly soluble salt?
g/L.What is the Ksp of this sparingly soluble salt?
A) 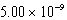
B) 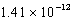
C) 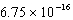
D) 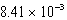
E) 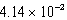
Correct Answer:
Verified
Q51: A solution containing 10.mmol of CO32- and
Q52: Which is the best colored indicator
Q53: What color change is exhibited by phenolphthalein
Q54: What is the solubility product expression for
Q55: Potassium hydrogen phthalate (KHP)is used to standardize
Q57: A 25.00-mL sample of propionic acid,HC3H5O2,of unknown
Q58: A solution containing 10.mmol of CO32-and 5.0
Q59: A 25.0 mL sample of 0.10
Q60: Consider the titration of 300.0 mL
Q61: Calculate the molar concentration of uncomplexed Zn2+(aq)in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents