Given the following equilibrium constants,
Cd(IO3) 2 Ksp = 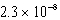
Cd(NH3) 42+ Kf = 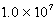
Determine K for the dissolution of the sparingly soluble salt Cd(IO3) 2 in aqueous ammonia (shown below) .Cd(IO3) 2(s) + 4NH3(aq)  Cd(NH3) 42+(aq) + 2IO3-(aq)
Cd(NH3) 42+(aq) + 2IO3-(aq)
A) 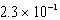
B) 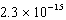
C) 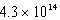
D) 4.3
E) 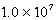
Correct Answer:
Verified
Q66: If the ratio of acid to base
Q78: What is the concentration of Cd2+(aq)in
Q79: What is the minimum mass of
Q80: A 4.0
Q81: Two important biological buffer systems control pH
Q82: For a monoprotic acid titration,the _ point
Q83: To make a buffer with a pH
Q84: Hyperventilation can cause your blood pH to
Q85: Given the two equilibria below, Q85: Why do salts containing basic anions have![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents