Which of the following statements concerning entropy change is/are true?
1) For a reversible process, 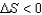 )
)
2) For a spontaneous process, 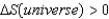 )
)
3) For a reversible process,such as a phase change, S = qrev/T .
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
Correct Answer:
Verified
Q2: A statement of the first law of
Q4: In which reaction is
Q5: If a chemical reaction occurs in a
Q6: Which of the following changes lead
Q9: Which of the following compounds has the
Q9: The following processes occur spontaneously at
Q10: When a chemical process occurs under standard
Q11: Which of the following linear chain alcohols
Q11: Which of the following statements is/are CORRECT?
1)Spontaneous
Q12: Which of the following statements concerning
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents