Which of the following reactions would be expected to have a positive entropy change, ∆rS° > 0?
1. 2 SO2(g) + O2(g) → 2 SO3(g)
2. Ba(OH) 2(s) → BaO(s) + H2O(g)
3. CO(g) + 2 H2(g) CH3OH( 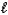 )
)
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3
Correct Answer:
Verified
Q11: Which of the following statements is/are CORRECT?
1)Spontaneous
Q12: Which of the following statements concerning
Q13: For a certain reversible process q
Q14: For which of the following reactions
Q15: The second law of thermodynamics states that
A)
Q17: Arrange the following reactions in order
Q18: What is the change in entropy for
Q19: Which of the following statements concerning entropy
Q20: Which of the following statements regarding the
Q21: Diluting concentrated sulfuric acid with water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents