Given the following,determine rG° at 298 K for the precipitation reaction,
Ag+(aq) +I-(aq) AgI(s) 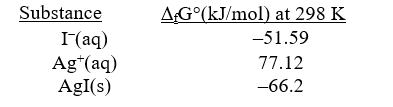
A) -91.7 kJ/mol-rxn
B) -40.7 kJ/mol-rxn
C) 91.7 kJ/mol-rxn
D) 40.7 kJ/mol-rxn
E) 62.5 kJ/mol-rxn
Correct Answer:
Verified
Q42: All of the following relationships are true
Q43: Estimate the boiling point of ethanol
Q44: At what temperatures will a reaction
Q45: Calculate
Q46: What is
Q48: What is
Q49: For which of the following substances is
Q50: For a reaction,
Q51: Thermodynamics can be used to determine all
Q52: Given that
S(g)+ O2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents