Write a balanced half-reaction for the reduction of hydrogen peroxide to water in an acidic solution.
A) 2 H2O2( 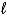 ) 2 H2O(
) 2 H2O( 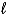 ) + O2(g)
) + O2(g)
B) 2 H2O2( 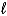 ) + 2e- 2 H2O(
) + 2e- 2 H2O( 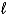 ) + O2(g)
) + O2(g)
C) H2O2( 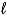 ) + 2 H+(aq) + 2 e- 2 H2O(
) + 2 H+(aq) + 2 e- 2 H2O( 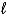 )
)
D) H2O2( 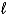 ) + 4 H+(aq) + 2 e- 2 H2O(
) + 4 H+(aq) + 2 e- 2 H2O( 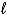 ) + H2(g)
) + H2(g)
E) H2O2( 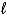 ) + 2 H+(aq) + 4 e- 2 H2(g) + O2(g)
) + 2 H+(aq) + 4 e- 2 H2(g) + O2(g)
Correct Answer:
Verified
Q7: Assuming the following reaction proceeds in
Q8: Assuming the following reaction proceeds in
Q9: The following reaction occurs spontaneously.
2 H+(aq)+
Q10: Balance the following oxidation-reduction occurring in
Q11: Write a balanced chemical equation for
Q13: Which of the following reactions would
Q14: Balance the following half-reaction occurring in
Q15: When the following oxidation-reduction reaction in
Q16: The following reaction occurs spontaneously.
2 Fe(s)+
Q17: Which of the following statements concerning a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents