Write a balanced chemical equation for the oxidation of Cd(s) by concentrated nitric acid,producing NO2(g) and Cd2+(aq) .
A) HNO3(aq) + Cd(s) Cd2+(aq) + NO2(g) + OH-(aq)
B) 2 HNO3(aq) + Cd(s) Cd2+(aq) + 2 NO2(g) + 2 OH-(aq)
C) HNO3(aq) + Cd(s) + H+(aq) Cd2+(aq) + NO2(g) + H2O( 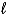 )
)
D) 4 HNO3(aq) + Cd(s) Cd2+(aq) + 2 NO2(g) + 2H2O( 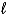 ) + 2 NO3-(aq)
) + 2 NO3-(aq)
E) HNO3(aq) + Cd(s) Cd2+(aq) + NO2(g)
Correct Answer:
Verified
Q1: How many electrons are transferred in the
Q2: Which of the following statements is true
Q3: When the following oxidation-reduction reaction in
Q5: Write a balanced half-reaction for the
Q6: All of the following statements concerning voltaic
Q7: Assuming the following reaction proceeds in
Q8: Assuming the following reaction proceeds in
Q9: The following reaction occurs spontaneously.
2 H+(aq)+
Q10: Balance the following oxidation-reduction occurring in
Q11: Write a balanced chemical equation for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents