Calculate 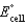 for the electrochemical cell below,
for the electrochemical cell below,
Ag(s) | AgCl(s) | Cl-(aq,1.0 M) || Cu2+(aq,1.0 M) | Cu(s)
Given the following standard reduction potentials.
Cu2+(aq) + 2 e- Cu(s) E = +0.337 V
AgCl(s) + e- Ag(s) + Cl-(aq) E = +0.222 V
A) -0.115 V
B) -0.107 V
C) +0.115 V
D) +0.452 V
E) +0.559 V
Correct Answer:
Verified
Q36: What is a correct cell notation
Q37: An SHE electrode has been assigned
Q38: According to the following cell notation,which species
Q39: Given:
2H+(aq)+ 2e-
Q40: The unit for electromotive force,emf,is the Volt.A
Q42: Calculate Ecell for the following electrochemical cell
Q43: What is the copper(II)-ion concentration at 25°C
Q44: For the electrochemical cell Zn(s)| Zn2+ ||
Q45: The following electrochemical cell has a
Q46: What is the pH of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents