When an aqueous solution of sodium sulfate is electrolyzed,what are the expected products?
Reduction Half-Reaction E° (V) 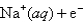 Na(s) -2.71
Na(s) -2.71
2H2O(l) + 2e- H2(g) + 2OH-(aq) -0.83
2H+(aq) + 2e- H2(g) 0.00
O2(g) + 4H+(aq) + 4e- 2H2O(l) 1.23
S2O82-(aq) + 2e- 2SO42-(aq) 2.01
A) Na(s) and H2(g)
B) H2(g) ,OH-(aq) ,O2(g) ,and H+(aq)
C) O2(g) ,H+(aq) ,and Na(s)
D) H2(g) ,OH-(aq) ,and Na(s)
E) H2(g) ,OH-(aq) ,and S2O82-(aq)
Correct Answer:
Verified
Q61: Given the following standard reduction potentials,
Pb2+(aq)+
Q62: What charge,in coulombs,is required to deposit
Q63: One kind of battery used in
Q64: What mass of chromium could be deposited
Q65: If
Q65: In an electrolytic cell,reduction occurs at the
Q67: The standard cell potential of the following
Q68: What half-reaction occurs at the cathode
Q70: If the value of E°cell is
Q71: How many moles of electrons are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents