What quantity,in moles,of oxygen is consumed when 369.3 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 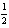 O2(g) H2O( ) ; rH° = -285.8 kJ/mol-rxn
O2(g) H2O( ) ; rH° = -285.8 kJ/mol-rxn
A) 0.6461 mol
B) 1.292 mol
C) 0.3869 mol
D) 1.146 mol
E) 0.1461 mol
Correct Answer:
Verified
Q24: What process is used to covert coal
Q25: What form of radiation is trapped by
Q26: Which of the following cannot be used
Q27: Which of the following is
Q28: The largest known source of tar sands
Q30: Methanol reacts with oils and fats to
Q31: The majority of crude oil ends up
Q32: Rank the three forms of coal,lignite,bituminous,and anthracite,from
Q33: Which of the following statements concerning
Q34: A byproduct of biodiesel production is
A) methanol
B)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents