Write a balanced chemical equation for the reaction of potassium and water.
A) K(s) + H2O( 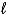 ) KO(s) + H2(g)
) KO(s) + H2(g)
B) 2 K(s) + 2 H2O( 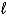 ) 2 KOH(aq) + H2(g)
) 2 KOH(aq) + H2(g)
C) 2 K(s) + 2 H2O( 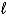 ) K2O2(s) + 2 H2(g)
) K2O2(s) + 2 H2(g)
D) 2 K(s) + H2O( 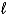 ) K2O(s) + H2(g)
) K2O(s) + H2(g)
E) 4 K(s) + 2 H2O( 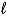 ) 4 KH(s) + O2(g)
) 4 KH(s) + O2(g)
Correct Answer:
Verified
Q32: Which of the following statements is INCORRECT?
A)
Q33: Which of the following statements concerning sodium
Q34: Which of the following statements are CORRECT?
1)Chili
Q35: The primary product of the reaction of
Q36: What mass of CO2 would be produced
Q37: How is sodium metal commercially produced?
A) By
Q40: What mass of MgO would be produced
Q41: Who developed the process for obtaining aluminum
Q42: What is the general valence shell electron
Q43: Nitric acid decomposes slowly in sunlight
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents