Based on the following data,what is the Cl-Cl bond energy?
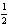 H2(g) +
H2(g) + 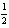 Cl2(g) HCl(g) ; rH = -92.31 kJ/mol-rxn
Cl2(g) HCl(g) ; rH = -92.31 kJ/mol-rxn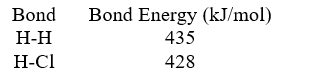
A) 432 kJ/mol
B) 236 kJ/mol
C) -236 kJ/mol
D) -421 kJ/mol
E) 421 kJ/mol
Correct Answer:
Verified
Q44: The active ingredients in "strike anywhere" matches
Q45: In what way is cryolite (Na3AlF6)used in
Q55: Phosphorus was first isolated in 1669 by
Q62: Which of the following statements is INCORRECT?
A)
Q63: Ammonium perchlorate can decompose violently according
Q64: Pure oxygen can be produced by
Q67: Anhydrous hydrogen fluoride is used in all
Q68: Arrange the following in order of increasing
Q75: Silicon is purified in a process called
Q77: What is the molecular formula of chlorous
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents