In an octahedral complex,electrons in 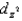 and
and 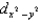
Orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
A) contain more electrons than the other three d orbitals.
B) each contain one unpaired electron.
C) are oriented directly toward the incoming ligand electron pairs.
D) are located a greater distance from the metal ion nucleus.
E) are roughly spherical in shape,much like an s orbital.
Correct Answer:
Verified
Q62: All of the following complexes will be
Q68: Determine the number of unpaired electrons in
Q70: One method of recovering metals from their
Q73: The spectrochemical series is an arrangement of
A)
Q73: Which of the following d-block ion
Q74: Which of the following cations would be
Q75: Which of the following statements concerning
Q76: As bound ligands,which of the following causes
Q77: The spectrochemical series is
CN- > NO2- >
Q78: Phenanthroline (C12H8N2)can bind to metal ions using
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents