What are the formal charges on boron and fluorine in the following structure? 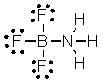
A) B = 1+, N = 1+
B) B = 1+, N = 1-
C) B = 1-, N = 1-
D) B = 1-, N = 1+
E) B = 1-, N = 0
Correct Answer:
Verified
Q16: Draw three constitutional isomers that have molecular
Q17: From the following, identify the item which
Q18: What is the correct Lewis dot structure
Q19: What is the formal charge on oxygen
Q20: Which of the following compounds are constitutional
Q22: Draw the Lewis structure for ozone, O3,
Q23: Which of the following structures have a
Q24: Which of the following structures have 1+
Q25: What is the correct Lewis structure for
Q26: What is the correct Lewis structure for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents