Which of the following is the correct representation of partial charges at the indicated atoms? 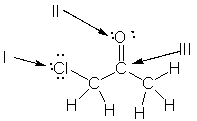
A) I = +; II = +; III = +
B) I = -; II = -; III = -
C) I = +; II = +; III = -
D) I = -; II = -; III = +
E) I = +; II = -; III = +
Correct Answer:
Verified
Q36: What is the formal charge on oxygen
Q37: What are the formal charges on boron
Q38: Which of the following structures have a
Q39: In an ammonium ion, nitrogen has a
Q40: Draw the Lewis structure for -CH2CN including
Q42: For the following compound identify the
Q43: Which of the following is the correct
Q44: For the following compound identify the
Q45: Which of the following is the most
Q46: Which of the following is the least
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents