For the following compound, identify each bond as polar covalent, nonpolar covalent or ionic and place a + on the most electropositive carbon. 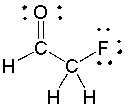
Correct Answer:
Verified
Q63: Which element has the electron configuration 1s2
Q64: Which element has the electron configuration 1s2
Q65: Ar, K+, and Cl- have equal numbers
Q66: The following ground state electron configuration violates
Q67: Which of the following principle states "When
Q69: What is the electronic configuration for the
Q70: Which of the following principle states that
Q71: What is the letter designation for the
Q72: What is the electronic configuration for the
Q73: Orbitals with the same energy are called
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents