What is the hybridization state of the oxygen atom in the following compound? 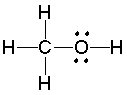
A) sp
B) sp2
C) sp3
D) sp3d
E) s2p
Correct Answer:
Verified
Q89: What is the hybridization state of the
Q90: Which of the following statement is incorrect,
Q91: Describe what happens to create a sp3
Q92: According to molecular orbital theory, the destructive
Q93: Which molecular orbitals are formed, when the
Q95: What is the hybridization state of the
Q96: Which of the bonding type has circular
Q97: According to molecular orbital theory, the constructive
Q98: Constructive interference of waves results in_.
A) a
Q99: Interaction of the following two atomic orbitals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents