The lone pairs of electrons of the nitrogen atom are located in which orbitals? 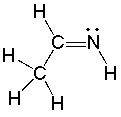
A) sp2
B) sp3
C) sp
D) s
E) p
Correct Answer:
Verified
Q107: Which of the following best describes the
Q108: How many s-sp2 sigma bonds are in
Q109: What is the hybridization state of the
Q110: The C-C sigma bond in ethyne
Q111: Which of the indicated carbon atoms have
Q113: How many pi bonds are present in
Q114: The bonds indicated by the arrow in
Q115: What is the hybridization state of the
Q116: Which of the following structures have carbon
Q117: What is the correct order of hybridization
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents