Which of the following compounds has a net dipole moment of zero? 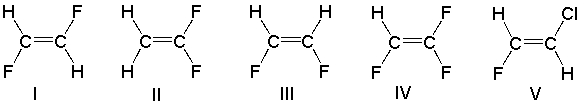
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q156: Draw the Lewis structure for SOCl2 and
Q157: What is the hybridization state and approximate
Q158: What is the approximate bond angle around
Q159: What is the hybridization state and molecular
Q160: What is the hybridization state and molecular
Q162: Which of the following compounds have the
Q163: Which of the following compounds has a
Q164: Which of the following statements best explains
Q165: BF3 has a no dipole moment. Draw
Q166: Which of the following compounds have a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents