What is the strongest intermolecular force present in the following compound? 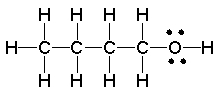
A) ion-dipole interactions
B) London dispersion forces
C) dipole-dipole interactions
D) hydrogen bonding
E) covalent bonding
Correct Answer:
Verified
Q170: Rank the following compounds in order of
Q171: Which of the intermolecular forces listed below
Q172: Which of the following compounds have the
Q173: What is the strongest intermolecular force present
Q174: Which intermolecular force is primarily responsible for
Q176: Which of the following compounds has a
Q177: Rank the following compounds in order of
Q178: Which of the following compounds have a
Q179: Which of the following compounds have the
Q180: Which of the following compounds have the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents