What is the formal charge on the indicated oxygen atom in the following compound? 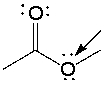
A) +1
B) +2
C) -1
D) -2
E) 0
Correct Answer:
Verified
Q90: What is the formal charge on the
Q91: What is the formal charge on the
Q92: How many lone pairs of electrons are
Q93: Which of the following compounds have +1
Q94: Diazomethane has the molecular formula CH2N2. Draw
Q96: What is the formal charge on the
Q97: What is the formal charge on the
Q98: How many lone pairs of electrons are
Q99: Which of the following compounds have +1
Q100: How many lone pairs of electrons are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents