The lone pair on nitrogen in the following compound is _______. 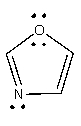
A) localized
B) delocalized
Correct Answer:
Verified
Q146: Draw the resonance hybrid of CH2CHCHCHCH2+.
Q147: Draw the resonance hybrid of C6H6.
Q148: Which of the following is/are the most
Q149: Draw the resonance hybrid for the following
Q150: Which of the following is/are the most
Q152: Which of the following is/are the most
Q153: Draw significant resonance structures for the following
Q154: What is the relationship between the following
Q155: Draw significant resonance structures for the following
Q156: The lone pair on nitrogen in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents