The following compound is best classified as a ____. 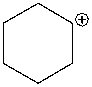
A) Brønsted-Lowry acid
B) Lewis acid
C) Brønsted-Lowry base
D) Lewis base
E) Both C & D
Correct Answer:
Verified
Q96: Which of the following solvents can not
Q97: Why is ethanol a better solvating solvent
Q98: For the following acid-base reaction, predict which
Q99: Determine if NaOH is a suitable reagent
Q100: Determine if H2O is a suitable reagent
Q102: Which of the following compounds is not
Q103: What is a cation?
A) a negatively charged
Q104: The following compound is best classified as
Q105: For the following reaction, identify the Lewis
Q106: For the following reaction, identify the Lewis
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents