Consider the following SN2 reaction, 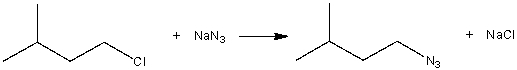 Assuming no other changes, what is the effect on the rate, if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled?
Assuming no other changes, what is the effect on the rate, if the concentration of both 1-chloro-3-methylbutane and NaN3 is doubled?
A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would reduce by half
Correct Answer:
Verified
Q20: What is the classification for the following
Q21: Provide the structure for (4R,8S)-4-iodo-2,2,8-trimethyldecane.
Q22: When drawing a curved arrow mechanism, the
Q23: What is the IUPAC name for the
Q24: Consider the following SN2 reaction, 
Q26: Which of the following is the rate
Q27: Which of the following is a reasonable
Q28: What is the IUPAC name for the
Q29: What is the correct structure for 2-bromo-3-methylbutane?
Q30: What is the IUPAC name for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents