For the following reaction, explain how you can use IR spectroscopy to monitor the progress of the reaction. 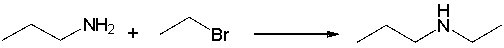
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q64: The separation of ions in the mass
Q65: In mass spectrometry, the tallest peak is
Q66: Mass spectrometry is primarily used to determine:
A)
Q67: Predict the product for the following reaction
Q68: Which of the following is not true
Q70: Which of the following is always true
Q71: In mass spectrometry using the electron impact
Q72: Which of the m/z values correspond to
Q73: Which of the following is true about
Q74: Which of the following is initially produced
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents