Which One of the Following Represents the Highest Energy -Antibonding Molecular Orbital of 1,3-Butadiene?
A) I
B) II
Which one of the following represents the highest energy -antibonding molecular orbital of 1,3-butadiene? 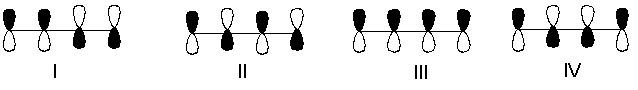
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q47: Provide the structure of the 1,2 addition
Q48: Predict the possible major products for the
Q49: How many
Q50: Which major product(s) is(are) formed in the
Q51: Identify the products of a reaction under
Q53: Which one of the following represents the
Q54: How many electrons does the LUMO of
Q55: Which one of the following represents the
Q56: How many electrons does the HOMO of
Q57: How many electrons does the HOMO of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents