A compound with molecular formula C8H14O4 exhibits a triplet at 1.3 (6H) , a singlet at 2.6 (4H) and a quartet at 4.2 (4H) in its 1H NMR spectrum. Its IR spectrum shows a strong absorption band near 1740 cm-1. What is the structure for this compound? 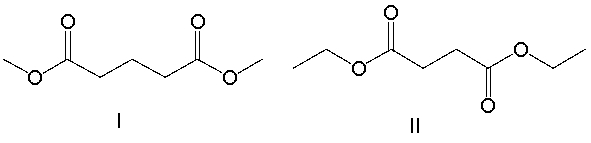
A) I
B) II
C) III
D) IV
E) none of these
Correct Answer:
Verified
Q134: Provide the reagents necessary to carry out
Q135: Propose a stepwise synthesis for N-propylbutanamide, using
Q136: Using ethyl 3-methylbutanoate as your only source
Q137: Provide the reagents necessary to carry out
Q138: Barbital, used as a sedative, is synthesized
Q139: Predict the product for the following reaction.
Q140: Predict the product for the following reaction
Q141: A compound with molecular formula C8H14O3
Q143: A compound with molecular formula C6H12O2
Q144: A compound with molecular formula C9H10O2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents